AUTHENTICITY
Matters
With great innovations,
comes many imitations.
MARKS OF AUTHENTICITYMARKS OFDON’T BE FOOLED BY A FAKEFAKEAUTHENTICITY

Ensure your treatment is performed by a certified Ultherapy PRIME provider, who has undergone rigorous training and is supported by Merz Aesthetics or an authorized distributor.
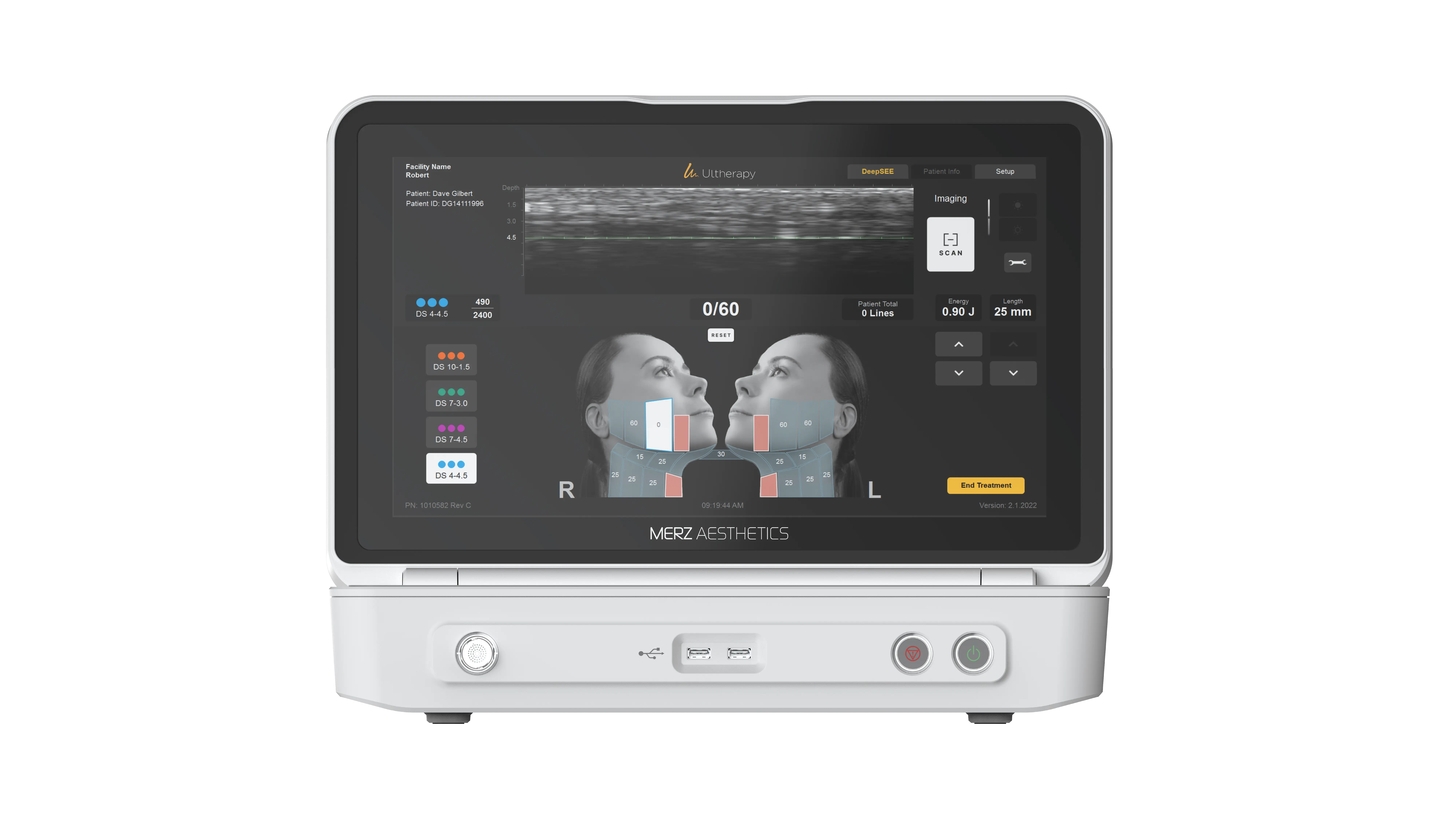
Confirm your treatment uses real-time visualization, not static or generic ultrasound images.

Request proof of authenticity such as the Ultherapy PRIME seal and certificate.
